corona tests

"Testing, testing, testing"
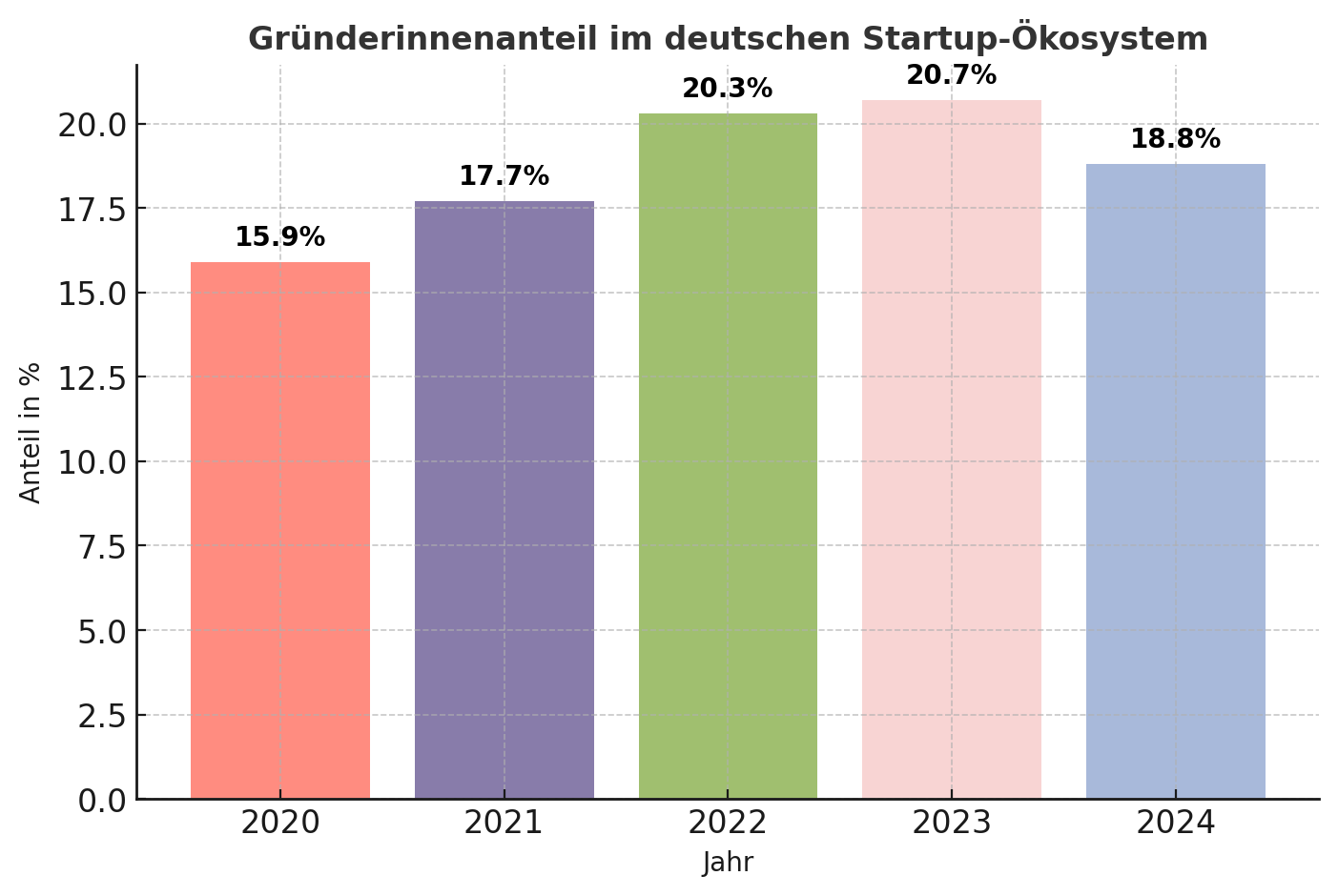
This is the new guiding principle of the Bavarian anti-corona strategy. From July 1, all people living in Bavaria will be able to get tested for coronavirus voluntarily and free of charge - even without symptoms. This is now possible as German laboratories have significantly increased their testing capacities in the fight against the coronavirus. In mid-June, around 100,000 samples were tested for the virus every day - in comparison, the daily laboratory capacity at the beginning of March was just over 7,000 tests.
Tests are currently being carried out in the laboratory using a polymerase chain reaction, or PCR for short. This involves amplifying the genetic material of the virus to such an extent that it can be detected, even if it only occurs in small quantities, according to the Federal Ministry of Health.
Such a test usually takes 4-5 hours, but valuable time elapses when the samples are sent to the specialist laboratories, time in which possible contact persons cannot yet be isolated and could infect other people.
Antibody test - the rescue?
For this reason, rapid antibody tests are currently being developed and are already being offered by various companies. However, these tests do not provide a snapshot of the infection, but instead detect an immune reaction against the pathogen. After the virus has been in the body for a few days, the human immune system produces antibodies that are easily detectable in the blood. The rapid antibody test should then provide this evidence. For this reason, the Federal Ministry of Health ordered millions of coronavirus antibody tests from the pharmaceutical company Roche at the beginning of May.
The large corporation is currently able to provide 5 million tests per month. However, the problem here is that a positive antibody test does not necessarily mean immunity. The test often also reacts positively to an infection with normal cold coronaviruses and falsely confirms a survived infection with the SARS-CoV-2 virus. Furthermore, this test only detects a survived infection and this 2-3 weeks after the actual infection, so acute cases cannot be isolated. Nevertheless, this is a possibility for rapid and comprehensive testing, but the possible risks and inaccuracies should not be neglected.

6 million in funding from the state of Baden-Württemberg
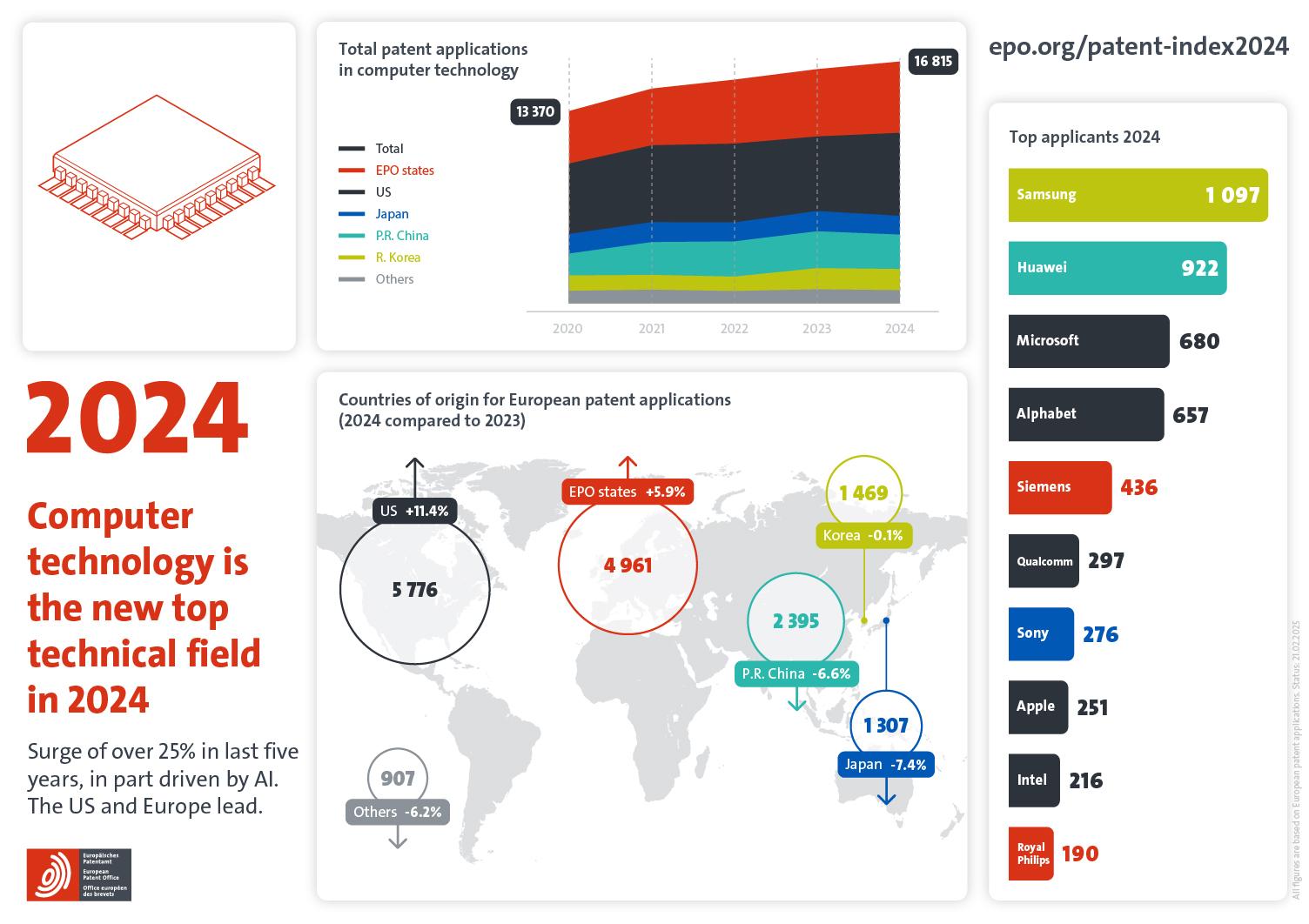
To make rapid testing more accurate, Spindiag is also currently working on a rapid test that should provide a result for a possible infection within 35 minutes. The Freiburg-based medical technology start-up, which was founded four years ago from the renowned Hahn-Schickard Institute for Microanalysis Systems, received six million euros from the state of Baden-Württemberg together with the research institute in April. The state intends to use this multi-million euro funding to drive forward the development of the rapid test. This should also be made possible by a fourth round of financing, including the participation of venture capitalist ThinkHealth Ventures, totaling 16.3 million euros.
Spindiag therefore has a good chance of successful development and market launch. So far, they have spent 15 years researching a machine that can detect multi-resistant germs within 30 minutes. This innovative test platform is now to be adapted to the SARS-COV-2 virus. It is a so-called cartridge test, in which a cartridge containing the virus genetic material is compared with the genetic material of the test subject. If the two match, the test is positive. In principle, rapid tests are carried out here, but with the precision of the PCR method. In this way, it would be possible to shorten the test duration from 4 hours to 30 minutes.

In addition, the device is able to analyze two cartridges simultaneously and could therefore provide 80 tests per device per day. According to co-founder and CEO Daniel Mark, the cost of a Sars-CoV-2 test is less than 50 euros, while the analyzer costs around 20,000 euros. The Freiburg-based start-up expects to enter the market in the third quarter of 2020.
Corona test in just 5 minutes
In addition to the classic PCR laboratory test, rapid antibody test and the cartridge test, there is another method of detecting the virus. A so-called antigen test, which is currently being researched by the Manizer start-up Digital Diagnostics. The start-up, which was founded in December, has just applied for special approval for its rapid coronavirus test from the Federal Institute for Drugs and Medical Devices. The antigen test is comparable to the PCr test, but promises a result within 5 minutes. With this accelerated procedure, it should be possible in future to process higher test numbers and make the infection process digitally recordable. However, the system is technically very complex and could be a little too expensive for mass screening; according to Digital Diagnostics, a test should cost just under 60 euros.

Nevertheless, we find this development remarkable and wish the start-ups continued success and hope for new opportunities in the fight against the coronavirus. That's it for our blog series on Pivotmaster during the crisis, we hope you were able to take away some information and inspiration. Stay healthy! Your Startbase team

Newsletter
Startups, stories and stats from the German startup ecosystem straight to your inbox. Subscribe with 2 clicks. Noice.
LinkedIn ConnectFYI: English edition available
Hello my friend, have you been stranded on the German edition of Startbase? At least your browser tells us, that you do not speak German - so maybe you would like to switch to the English edition instead?
FYI: Deutsche Edition verfügbar
Hallo mein Freund, du befindest dich auf der Englischen Edition der Startbase und laut deinem Browser sprichst du eigentlich auch Deutsch. Magst du die Sprache wechseln?